Hello, and welcome back to this week’s edition of the Gist Weekly. We immensely appreciate your continued readership and invite you to forward this email to friends and colleagues—please encourage them to subscribe.
In the News
What happened in healthcare recently—and what we think about it.
- All members of CDC vaccine advisory panel dismissed. In an opinion piece published in The Wall Street Journal on Monday, Health and Human Services Secretary Robert F. Kennedy Jr. revealed that he was “retiring” all 17 members of the Advisory Committee on Immunization Practices (ACIP). The ACIP is convened by the Centers for Disease Control and Prevention (CDC) to review evidence and make recommendations on vaccines that inform the vaccine schedule and coverage. Kennedy said ACIP committee members had conflicts of interest but did not cite specific examples. On Wednesday, Secretary Kennedy announced 8 new members for the panel, which is scheduled to meet later this month, with discussions on HPV and Covid-19 vaccines expected.
- The Gist: While Secretary Kennedy stated he made this move to “restore public trust in vaccines,” this action also could sow further doubt in public health and has been met with skepticism even from some Republicans. In his announcement, Secretary Kennedy stated, “without removing the current members, the current Trump administration would not have been able to appoint a majority of new members until 2028,” a statement that critics said politicizes a committee that is supposed to be apolitical. The move pushes the boundaries of a promise Kennedy made to Senator Bill Cassidy to secure his support during confirmation proceedings. While Secretary Kennedy’s new picks have relevant experience, some appear to share his skeptical views of vaccines. For example, Dr. Robert Malone has stated that getting the Covid-19 vaccine put people who already had Covid at higher risk for side effects, a claim that was disputed by doctors and healthcare professionals, and Vicky Pebsworth has served on the board of the National Vaccine Information Center, which “warns against vaccine risks.” The continued politicization of vaccines may confuse patients, who must parse through conflicting messaging on vaccines.
- States seeking to block sale of personal genetic information. On Monday, 27 states and the District of Columbia filed a lawsuit in bankruptcy court to try to halt the sale of personal genetic information without consent from every customer. Per a press release regarding the case, “states argue that customers should have the right to control such deeply personal information and that it cannot be sold like ordinary property.” Regeneron Pharmaceuticals is seeking to buy 23andMe and said it would honor all existing consents, privacy policies and statements, terms of services and notices. A court-appointed, independent consumer privacy ombudsman was due to report how the proposed sale could affect consumer privacy by Tuesday.
- The Gist: The sale of 23andMe poses privacy and consent questions that our healthcare and legal systems have never had to ponder. Despite the clear healthcare implications, there are fewer legal protections for consumers’ data because 23andMe is a direct-to-consumer company and genetic testing is not considered a traditional medical test. The states’ privacy argument is compelling. Consumers consented toa direct-to-consumer company with a research arm to own their data, not a pharmaceutical company. However, the logistics of getting explicit consent from every single customer appear near impossible. Genetic information is powerful; there is still so much we do not know about DNA that could inform future innovation. Consumer privacy appears to be front-of-mind for the parties involved, as the court could demand additional privacy requirements as a condition for the deal’s approval.
- FDA to leverage AI in drug approval process. The Food and Drug Administration (FDA) will alter its processes to use artificial intelligence (AI) in the drug and device approvals processes to “radically increase efficiency.” The change was announced on Wednesday in an article by commissioner Marty Makary and a senior FDA official, Vinay Prasad. The article, published in JAMA, outlined new FDA strategic priorities. It said the agency will also use AI to review chemicals that are in the United States’ food supplies but not on those of other developed nations. The announcement comes on the heels of the FDA’s introduction of Elsa, an AI tool like ChatGPT.
- The Gist: The FDA is wrestling with the same question that confronts many companies: how to use burgeoning AI technology. AI has the potential to increase efficiency, especially for the FDA, given its loss of staff, but there are doubts as to whether AI has a sufficient degree of expertise. Elsa’s rollout was met with mixed reviews, reportedly “helpful but far from transformative” and even hallucinating. For now, AI is being used as a sort of “first pass,” but as a key healthcare regulator, FDA’s stakes are high should something go wrong.
Plus—what we’ve been reading.
- Quality of life takes center stage for pediatric cancer survivors living longer. Published several weeks ago in The Washington Post, this article is the account of a parent who reflects on her daughter’s life as a high-risk neuroblastoma cancer survivor. She draws attention to the growing population of pediatric cancer survivors—nearly half a million children in the United States, 85% of whom live at least 5 years after their initial diagnosis—and what this means for their long-term care. Experts note that caring for young survivors, who must navigate persistent, lifelong health challenges and chronic health conditions, is nuanced and different from adult cancer survivors. The author details the progress toward the guidelines, tools and treatments and their impact on improving post-treatment complications and informing survivorship care plans. She advocates for a continued commitment to improving outcomes and quality of life, recognizing the role of funding, research and healthcare delivery.
- The Gist: Pediatric cancers are rare, yet they’re the leading cause of death by disease in patients under 20. The decline in pediatric cancer mortality and the growing number of pediatric cancer survivors is remarkable, especially because of how limited federal research funding is--only 4% of federal cancer funds go to pediatric cancer research. Advances are changing how we think about cancer care long-term—with lifelong care implications and chronic risk factors, financial toxicity and quality of life taking center stage. Rethinking long-term cancer survivorship will require greater investment in treatment and integration across pediatric and adult care services to improve quality of life for pediatric survivors, a strategy already utilized by more than 100 provider organizations.
Graphic of the Week
A key insight illustrated in infographic form.
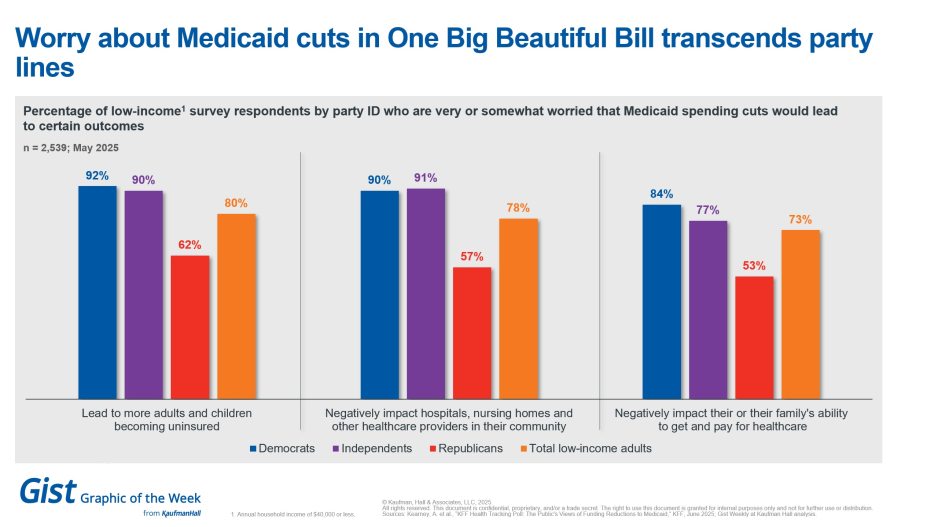
Bipartisan worry about Medicaid cuts
As the One Big Beautiful Bill Act makes its way through the Senate, this week’s graphic features new survey data from KFF on how the public currently feels about potential Medicaid cuts. Regardless of party identification, more than half of low-income adults surveyed are worried about how Medicaid cuts could increase the uninsured rate, harm providers in their communities and negatively impact their or their family’s ability to pay for care. Democrats and independents are more worried compared with Republicans, with 9 in 10 Democrats and independents expressing concern about the impacts on the uninsured rate and providers. These new data are another illustration of Medicaid’s enduring bipartisan popularity with voters and reminds Republican lawmakers of the potential political cost of the bill.
This Week at Kaufman Hall
What our experts are saying about key issues in healthcare.
Healthcare for an individual and for populations should be optimized for the long term, ideally for life. But our basic health insurance product structure has been an annual one, incentivizing optimization primarily over the one-year timeframe, and then “rinse and repeat” the same cycle every year.
In a new blog post, Joyjit Saha Choudhury describes what would be required to move to a multi-year horizon for health insurance. Although multi-year products will be a tough nut to crack, a big prize could await those that can pull off this innovation as the U.S. population ages and financing for healthcare gets even more strained over time.
On Our Podcast
The Gist Healthcare Podcast—all the headlines in healthcare policy, business, and more, in ten minutes or less every other weekday morning.
Last Monday, Modern Healthcare’s Michael McAuliff joined host J. Carlisle Larsen to talk about proposed cuts in federal healthcare spending, primarily to Medicaid, the reconciliation bill that has been dubbed the One Big Beautiful Bill Act of 2025.
This Monday, JC is joined by Sylvia Owusu-Ansah, MD, associate professor of pediatrics and emergency medicine at the University of Pittsburgh School of Medicine and the director of EMS at UPMC Children’s Hospital-Pittsburgh Dr. Owusu-Ansah recently served as a medical consultant on the hit HBO Max series The Pitt and tells JC about her experience.
To stay up to date, be sure to tune in every Monday, Wednesday, and Friday morning. Subscribe on Apple, Spotify, Google, or wherever fine podcasts are available.
As a quick programming note, the Gist Weekly will be off next week. We look forward to delivering the latest industry insights on June 27. In the meantime, check out our Gist Weekly archive if you’d like to peruse past editions. We also have all of our recent “Graphics of the Week” available here.
Best regards,
The Gist Weekly team at Kaufman Hall